Articles from Kerecis

Kerecis, the company pioneering the use of sustainably sourced fish skin and fatty acids in cellular therapy and tissue regeneration, welcomes the recent decision by the Centers for Medicare & Medicaid Services (CMS) to postpone the effective date of the proposed Local Coverage Determination (LCD) for Skin Substitute Grafts/Cellular and Tissue-Based Products. The new implementation date is January 1, 2026.
By Kerecis · Via Business Wire · April 14, 2025

Iceland’s President, Halla Tomasdottir, presented the Asa Gudmundsdottir Wright Award at a ceremony at the presidential residence. This year’s recipients are G. Fertram Sigurjonsson, Founder and CEO of Kerecis, and Jon Atli Benediktsson, Rector of the University of Iceland. The award honors individuals who have made significant contributions to science and innovation in Iceland.
By Kerecis · Via Business Wire · February 14, 2025

Kerecis, the company pioneering the use of sustainably sourced fish skin and fatty acids in cellular therapy and tissue regeneration and protection, today announced the availability of SurgiClose® Silicone, which combines a fish-skin graft and silicone backing for efficient treatment of surgical and trauma wounds.
By Kerecis · Via Business Wire · December 27, 2024
Kerecis, the pioneer in the use of fish skin and fatty acids for tissue regeneration and protection, today announced Medicare coverage approval of two of its products by the U.S. Centers for Medicare & Medicaid Services (CMS) in a new Local Coverage Determination (LCD).
By Kerecis · Via Business Wire · November 14, 2024
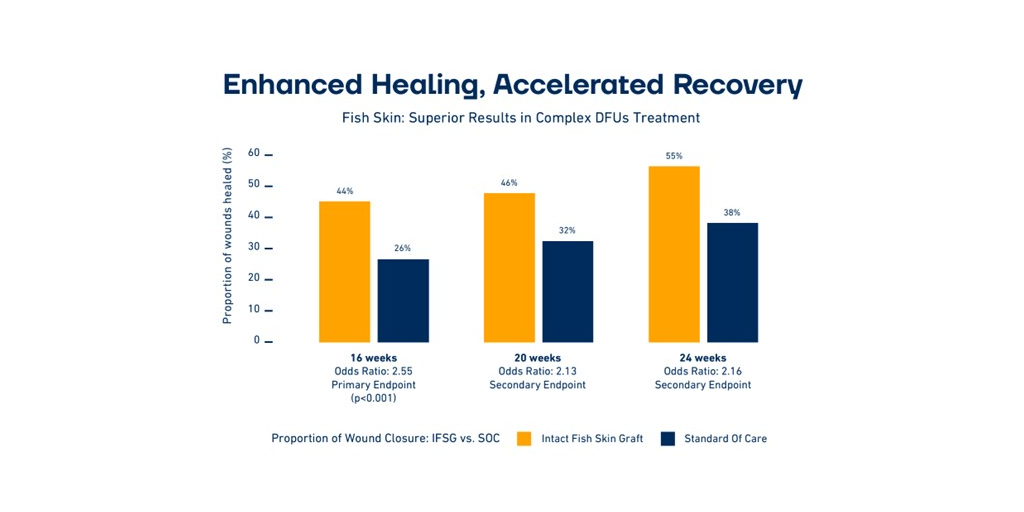
Kerecis, the pioneer in the use of fish-skin and fatty acids for tissue regeneration and protection, today announced the publication of breakthrough study results in the New England Journal of Medicine Evidence. The study was conducted on a sample of 255 patients at 15 care centers across four countries. The study is the largest randomized controlled trial published to date on the efficacy of using biologic skin substitutes to treat foot ulcers with exposed bone and tendons. The study demonstrates that fish-skin grafts significantly outperform the standard of care in closing University of Texas grade 2 and 3 diabetic foot ulcers.
By Kerecis · Via Business Wire · October 4, 2024
Kerecis, the pioneer in the use of fish-skin and fatty acids for tissue regeneration and protection, is a high-profile participant and presenter at this year’s Symposium on Advanced Wound Care (SAWC). Attendees are discovering the significant science supporting fish-skin graft’s superior ability to treat difficult-to-close wounds and accelerate recovery. SAWC is currently taking place in Las Vegas through October 5, 2024. The Kerecis booth is number 517.
By Kerecis · Via Business Wire · October 3, 2024
Kerecis, the company pioneering the use of sustainably sourced fish-skin and fatty acids in cellular therapy, tissue regeneration and protection, has received approval from Palmetto GBA for the Kerecis Shield™ private doctors’ clinics fish-skin product range. The approval makes the product eligible for Medicare Part B coverage across Alabama, Georgia, North Carolina, South Carolina, Tennessee, Virginia and West Virginia.
By Kerecis · Via Business Wire · July 16, 2024
Kerecis, the company pioneering the use of sustainably sourced fish skin and fatty acids in cellular therapy and tissue regeneration and protection, today announced the availability of Shield Spiral, an extension of the Kerecis Shield silicone fish-skin combination product range.
By Kerecis · Via Business Wire · July 5, 2024

Kerecis, the pioneer in the use of fish skin and fatty acids for tissue regeneration and protection, will unveil its latest scientific and clinical updates, including trial results, at the upcoming Symposium on Advanced Wound Care (SAWC) Spring. The company will offer educational symposiums, a hands-on skills lab, an interactive poster session, a cultural event, and a panel discussion of female medical professionals. Kerecis will exhibit at booth 419 at SAWC, which will take place from May 14 to 18 in Orlando, Florida.
By Kerecis · Via Business Wire · May 14, 2024

The European Wound Management Association (EWMA) presented Fertram Sigurjonsson, founder and CEO of the medical-fish-skin company Kerecis, with the President’s Wound Care Entrepreneur of the Year Award. The award was announced at the annual EWMA Innovation Forum.
By Kerecis · Via Business Wire · May 3, 2024

Kerecis, the company pioneering the use of sustainably sourced fish skin and fatty acids in cellular therapy and tissue regeneration and protection, will present two symposiums and 27 abstracts on its intact medical fish skin at the European Wound Management Association (EWMA) this week. The company will also host a medical education event on the utilization of fish skin grafts on Wednesday, May 1. Finally, Kerecis’ founder and CEO will summarize the company’s journey at the EWMA Innovation Forum Thursday, May 2. Kerecis will be exhibiting at booth Nr. G30 at EWMA, which takes place in London from May 1 to May 3.
By Kerecis · Via Business Wire · May 1, 2024

Kerecis, the medical-fish-skin company, has been named Iceland’s Export Company of the Year. The President of Iceland, Gudni Th. Johannesson, presented the award to Kerecis’ founder and CEO Fertram Sigurjonsson at a ceremony at the President's residence. Former and current Kerecis staff, board members, and investors attended.
By Kerecis · Via Business Wire · March 13, 2024

Kerecis, the company pioneering the use of sustainably sourced fish skin and fatty acids in cellular therapy and tissue regeneration and protection, today announced the availability of Shield Standard, which combines a fish-skin graft and silicone backing for efficient treatment of acute and chronic wounds.
By Kerecis · Via Business Wire · February 15, 2024
SAWC – Kerecis today announced the publication of a multi-disciplinary clinical consensus about the utilization of fish skin grafts on complex wounds. The consensus paper will be presented at a CME/CPME/CE-accredited symposium, which will take place Saturday, November 4, from 11:30 am to 12:30 pm in Milano VII-VIII, Caesars Palace, Las Vegas at the SAWC wound conference. Pre-registration is not required for conference attendees. Kerecis, which is pioneering the use of sustainably sourced fish skin and fatty acids in cellular therapy and tissue regeneration and protection, is exhibiting at booth 517.
By Kerecis · Via Business Wire · November 3, 2023

Kerecis® Shield™, the new fish-skin wound-treatment product family for private doctors’ clinics, has been approved by multiple leading Medicare Administrative Contractors (MACs). This means that physicians operating in private clinics will not need to submit invoices for reimbursement, improving efficiency by eliminating a step in the reimbursement process.
By Kerecis · Via Business Wire · August 25, 2023

Kerecis®, the company pioneering the use of fish skin and fatty acids for tissue regeneration and protection, is donating its GraftGuide® fish-skin burn product for victims of the fires on Maui, Hawaii. Qualified medical personnel wanting to get fish-skin burn-treatment products for their patients should call 703-287-8752 or email wildfires@kerecis.com. Kerecis is dispatching a specialist to train doctors who would be using the product for the first time.
By Kerecis · Via Business Wire · August 14, 2023

Nordic Scaleup Summit-- Kerecis, the company pioneering the use of sustainably sourced fish skin and fatty acids in cellular therapy and tissue regeneration and protection, today received the Nordic Scaleup Award for 2023.
By Kerecis · Via Business Wire · May 10, 2023
SAWC – Kerecis® today announced MariGen® Shield, which integrates the company’s proven fish-skin graft with a silicone contact layer for treating chronic and complex wounds. The medical-fish-skin company also announced the results of a clinical study comparing the effectiveness of the Kerecis fish-skin grafts to a standard of care for diabetic foot ulcers. Both announcements were made at the Symposium on Advanced Wound Care (SAWC). Kerecis, which is pioneering the use of sustainably sourced fish skin and fatty acids in cellular therapy and tissue regeneration and protection, is exhibiting at booth 225.
By Kerecis · Via Business Wire · April 27, 2023

Kerecis announced today that, over the past year, 63 million more Americans became eligible for insurance coverage for the company’s fish-skin treatments, an increase of more than 70%. This is the result of more private insurance companies deciding to cover the Kerecis fish-skin technology, in addition to previously existing Medicare coverage. This milestone means that about 150 million Americans — about 45% of the country’s population — can now enjoy the benefits of the patented fish-skin treatment.
By Kerecis · Via Business Wire · April 18, 2023

Kerecis® has been included in the FT1000 list of Europe’s fastest growing companies. The medical-fish-skin company ranked fifth in the healthcare and life sciences category–and 246th overall–in the seventh annual list compiled by the “Financial Times.” This is the first time that Kerecis has been included in the prestigious list.
By Kerecis · Via Business Wire · March 21, 2023

Kerecis, the company pioneering the use of fish skin and fatty acids for tissue regeneration and protection, today announced the release of two new products for the burn market, GraftGuide Mano™ and GraftGuide Micro™.
By Kerecis · Via Business Wire · January 20, 2023

Kerecis, the company pioneering the use of fish skin and fatty acids for tissue regeneration and protection, today announced that the company will be attending the Biotech Showcase™ 2023 and the 41st Annual J.P. Morgan Healthcare Conference, which will take place in San Francisco the week of January 9, 2023.
By Kerecis · Via Business Wire · January 3, 2023

Kerecis® today announced that it completed its $100 million Series D financing that was led by KIRKBI, the family holding and investment company behind the LEGO Group. Kerecis is the company pioneering the use of sustainably sourced fish skin and fatty acids in cellular therapy and tissue regeneration and protection globally. The funding round was supported by several existing shareholders, including Silicon Valley’s Emerson Collective and the Icelandic pension funds BRU and LSV. The funding round also included conversion of existing convertible debt to equity and new and extended debt facilities.
By Kerecis · Via Business Wire · August 1, 2022

Atrium Health’s Sanger Heart & Vascular Institute has received a Center of Excellence designation from Kerecis®, the company pioneering the use of fish skin and fatty acids in cellular therapy, tissue regeneration and protection. Sanger Heart & Vascular Institute surgeon Dr. Hector Crespo Soto is the first physician in the world to use SurgiBind implantable reinforcement fish-skin commercially. With this designation, Crespo Soto and the team at Sanger Heart & Vascular Institute can continue to lay the groundwork for centers around the country to utilize innovative technology to improve patient care and quality of life.
By Kerecis · Via Business Wire · July 6, 2022

Kerecis®, the company pioneering the use of fish skin and fatty acids in cellular therapy, tissue regeneration and protection, today announced MariGen™ Expanse, the newest addition to Kerecis’ product offerings for chronic wound management. The announcement was made at the Symposium for Advanced Wound Care (SAWC) meeting, which is taking place in the Phoenix Convention Center from April 6 to 10, 2022. Kerecis is exhibiting at booth 325.
By Kerecis · Via Business Wire · April 8, 2022

Kerecis, the company pioneering the use of fish skin and fatty acids in cellular therapy, tissue regeneration and protection, announced today that the company‘s products have been added to the Healogics iSupply program. This makes Kerecis medical-fish-skin products available to Healogics Wound Care Centers®, expanding the options to treat chronic, non-healing wounds. The announcement was made at the Symposium for Advanced Wound Care (SAWC) Spring meeting. Kerecis is pioneering the use of fish skin and fatty acids for tissue regeneration and protection globally. Healogics is the leading provider of world-class wound care services in the United States.
By Kerecis · Via Business Wire · April 5, 2022

Kerecis and Arctic Circle announced today that they will partner to increase attention on the rapid changes in the Arctic. The partnership will allow Kerecis to influence and participate in the organization’s dialogue on the future of the “blue economy” in the region. Arctic Circle, the largest international network on the Arctic, will in turn gain the benefit of Kerecis’ insights into how to protect and strengthen this important ecosystem.
By Kerecis · Via Business Wire · March 30, 2022

Kerecis, the company pioneering the use of fish skin and fatty acids in cellular therapy, tissue regeneration and protection, today announced that Fertram Sigurjonsson, Kerecis Founder and CEO, and Mike Cadigan, Chief Financial Officer, will participate in meetings at SVB Leerink Annual Global Healthcare Conference on Monday and Tuesday, February 14 and 15, 2022.
By Kerecis · Via Business Wire · February 14, 2022

Kerecis®, the company pioneering the use of fish skin and fatty acids in cellular therapy, tissue regeneration and protection, has received authorization from the FDA to market Kerecis Omega3 SurgiBind™. The product, which is available now in the U.S., is an implantable fish-skin graft for use in plastic and reconstructive surgery. The product is indicated for implantation to reinforce soft tissue where weakness exists, in patients requiring soft tissue repair, or reinforcement in plastic or reconstructive surgery.
By Kerecis · Via Business Wire · October 19, 2021

A study published in the peer-reviewed journal Wounds reports statistically significantly higher healing rates for diabetic foot ulcers (DFUs) treated with Kerecis® Omega3 fish skin compared to DFUs treated with Fibrocol, a collagen-alginate dressing. Of the 64 patients enrolled in the study, 15 healed more than 20% during the screening period or dropped out. The remaining 49 were randomized and included in the intent-to-treat analysis. Of the patients treated with the Kerecis product, 67% had healed after 12 weeks of treatment versus 32% in the control group. The p‑value was .0152, which is considered statistically significant.
By Kerecis · Via Business Wire · September 20, 2021

Ernst & Young LLP (EY US) yesterday announced that Kerecis Founder and CEO Fertram Sigurjonsson was named an Entrepreneur Of The Year® 2021 Mid-Atlantic Award winner. The Entrepreneur Of The Year Awards program is one of the preeminent competitive awards for entrepreneurs and leaders of high-growth companies. The award recognizes those who are unstoppable entrepreneurial leaders, excelling in talent management; degree of difficulty; financial performance; societal impact and building a values-based company; and originality, innovation and future plans. Sigurjonsson was selected by an independent panel of judges, and the award was announced during the program’s virtual awards gala on August 3, 2021.
By Kerecis · Via Business Wire · August 4, 2021

Ernst & Young LLP (EY US) has named G.F. Sigurjonsson, founder and president of Kerecis, one of the finalists for the Entrepreneur Of The Year® 2021 Mid-Atlantic Award. Now in its 35th year, the Entrepreneur Of The Year is one of the preeminent competitive award programs for entrepreneurs and leaders of high-growth companies.
By Kerecis · Via Business Wire · June 11, 2021
Kerecis, the company pioneering the use of fish skin and fatty acids for tissue regeneration and protection, today announced the release of Kerecis® Omega3 MicroGraft. The easy-to-use product is intact fish skin divided into fragments, which mold into wound beds. Kerecis® Omega3 MicroGraft is appropriate for treating complex trauma and uneven wounds in operating rooms, wound centers and doctors’ offices.
By Kerecis · Via Business Wire · June 2, 2021

Kerecis®, the company that is pioneering the use of fish skin in tissue regeneration and wound care, today announced its new product, Kerecis Omega3 GraftGuide™, at the 43rd Annual John A. Boswick Burn & Wound Care Symposium.
By Kerecis · Via Business Wire · April 20, 2021
